Research cluster: Autotrophe Produktion / Subproject |
| Subproject 3: Stable production of variants of cyanophycin |
| Project time: 01.09.09-28.02.13 |
| Project leader: |
| Prof. Dr. Alexander Steinbüchel, Westf. Wilhelms-Universität Münster |
| Cyanophycin, also referred to as multi-L-arginyl-poly-[aspartic acid], represents a non-ribosomally synthesized polyamide usually consisting of a poly(aspartic acid) backbone with arginine residues linked to the β-carboxyl group of each aspartic acid moiety by the α-amino group (Fig.1). |
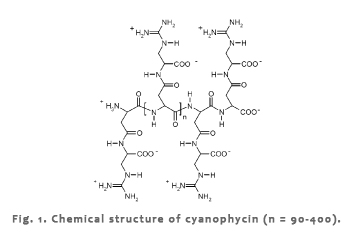 |
| For cyanophycin synthesis only one enzyme is required, which is referred to as cyanophycin synthetase (CphA). Applications for several degradation products of cyanophycin are of special industrial and medical interest. For example, the polymer can function as an environment-friendly substitute for non-biodegradable poly(acrylic acid) or it could be a source of Asp-Arg or Asp-Lys dipeptides, which are used in pharmaceutical products. Especially for the synthesis of new dipeptides production of new cyanophycins consisting of aspartic acid with citrulline, lysine or ornithine, respectively, as side chain instead of arginine is interesting. In addition to Asp-Arg, dipeptides consisting of Asp-Lys, Asp-Cit, or Asp-Orn would broaden the application range of these compounds. To obtain different variants of cyanophycin concerning its composition, the metabolism of R. eutropha will be engineered applying metabolism-based plasmid addiction systems. These systems have been recently approved in R. eutropha and E. coli and provide a tool for stable maintenance of plasmids without using external selection pressure like antibiotics and increased cyanophycin production by recombinant expression of appropriate cyanophycin synthetases. |
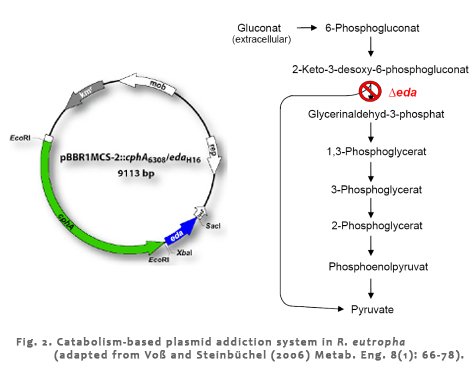 |
References: |
| Fleige C, Kroll J, Steinbüchel A (2011) Establishment of an alternative phosphoketolase-dependent pathway for fructose catabolism in Ralstonia eutropha H16. Appl Microbiol Biotechnol 91:769-776 |
| Kroll, J., Steinle, A., Reichelt, R., Ewering, C. and Steinbüchel, A. (2009) Establishment of a novel anabolism-based addiction system with an artificially introduced mevalonate pathway: complete stabilization of plasmids as universal application in white biotechnology. Metab. Eng. 11: 168-177. |
| Steinle, A., Bergander, K. and Steinbüchel, A. (2009) Metabolic engineering of Saccharomyces cerevisiae for production of novel cyanophycins with an extended range of constituent amino acids. Appl. Environ. Microbiol.75: 3437-3446. |
Funded by


New Brochure: Facetten der Genomforschung
Wissenschaftler entschlüsseln die Baupläne des Lebens (german; pdf; 12,3 Mb)
Wissenschaftler entschlüsseln die Baupläne des Lebens (german; pdf; 12,3 Mb)

